
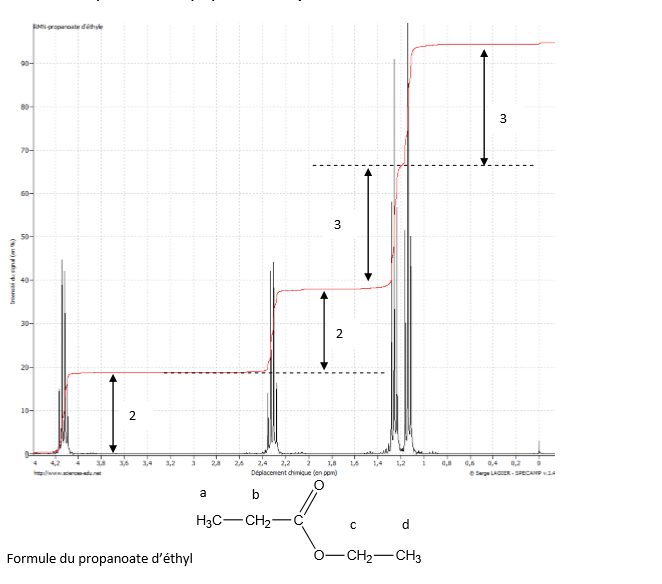
Ceci provoque un déroulement du complexe a partir d'un côté. La première et la seconde protonation du complexe se font préférentiellement et respectivement sur les atomes d'azote terminaux et centraux et sur le même côté du complexe provoquant ainsi la rupture respective des liaisons métal–azote. Dans le cas d'une complexation 1:1 avec le Pb(II) les constantes d'équilibre suivantes ont été obtenues pour la protonation (acide triéthylènetétraaminehéxaacétique ≡ H 6A) Dans le complexe non protoné, on considère que les quatre liaisons métal–azote sont coplanaires (ou presque coplanaires), que les groupes carboxylates centraux sont coordonnés au-dessus et en dessous du plan et que les groupes carboxylates terminaux n'interviennent que très peu dans les liaisons coordonnées. a été utilisée pour déterminer les sites préférentiels de protonation dans le TTHA. Rapid interconversion between configurations in which unwrapping and rewrapping occurs first from one side of the molecule and then from the other leads to simplified n.m.r.
This causes partial unwrapping of the complex from one side. The first and second protonations of the complex occur preferentially at the terminal and center nitrogen atoms, respectively, on the same side of the complex, accompanied by breaking of the respective metal–nitrogen bonds. For its 1:1 complex with Pb(II) the following equilibrium constants for protonation were obtained (triethylenetetraaminehexaacetic acid ≡ H 6A) The non-protonated complex is considered to have four coplanar (or nearly coplanar) metal–nitrogen bonds with the center carboxylate groups coordinated above and below this plane, and with the terminal carboxylate groups playing only a small part in the coordinate bonding. However more information can be obtained using variable field NMR relaxometry.Nuclear magnetic resonance (n.m.r.) spectroscopy was used to determine the preferred protonation sites in TTHA. Low field proton relaxometry, with water as probe molecule, is efficient to probe the porosity of porous polymer particles. Now, we want to push the limits of our methods in order to be able to follow in situ and at the atomic scale the processes occurring during drug delivery from a MOF in a biological medium.Įvolution of the MOF NP porosity during drug delivery is also a parameter to take into account. The NMR methods are nowadays well established for the study of host-guest interactions in porous solids. Analysis of solid-state NMR spectra provides information about the nature and quantity of the species, their coordination, dynamics and potential interactions. In this context, nuclear magnetic resonance (NMR), a non-destructive local technique, appears ideally suited to study these MOF NPs that lack long-range order. If usual techniques (HPLC, etc) can provide data on the delivery, none of them allows an accurate description at the atomic scale of the nanoMOF and active principle ingredient evolution during degradation, the nature of the formed species and their influence upon drug release. The control over the MOF NP degradation in biological media is a key parameter as it in turn allows a better control of the drug delivery kinetics. The high porosity of MOFs, their high specific surface and unrivalled versatility in terms of composition and easy functionalization, are the reasons why these materials are particularly appealing systems for loading of large amounts of therapeutic molecules. La spectroscopie de RMN haute rsolution du LCR: problmes. Nanoparticles (NPs) based on porous metal-organic frameworks (MOFs) are of growing interest in medicine, with applications for controlled drug delivery, targeting of diseased tissues/organs or in imaging. Analysis of plasma lipids by NMR spectroscopy: application to modifications induced by. Project leader: Charlotte Martineau-Corcos


 0 kommentar(er)
0 kommentar(er)
